Presenting the Nutrition Declaration and Ingredients List for Wines
A Quick Guide
Updated July 1, 2024
This page aims to provide a guide on how to present the Nutrition Declaration and Ingredients List on the label of wines and other grapevine products when using a QR Code.
Summary of Regulation Changes
According to the latest EU Regulations (Regulation (EU) No 1308/2013), all wines that are produced on or after 8 December 2023 and are sold in the EU market, must include the nutrition declaration and the list of ingredients as part of the presentation of the compulsory particulars.
Any wines produced before that date may continue to be placed in the market following the labelling requirements applicable before 8 December 2023, until stocks are exhausted.
Producers can disclose the above information either on the label attached to the container OR via electronic means (e.g. QR Code) provided that they follow specific requirements set by the EU regulations for each case.
The new regulations apply also to wines imported in EU, transported in bulk and all other labelled packaged wine products.
Producers will be also required to include the list of ingredients as part of the description of the imported product in VI-1 certificates.
Online stores must also provide the nutrition declaration and the list of ingredients to the customers.
If I decide to present the Nutrition Declaration and the List of Ingredients electronically via a QR Code, what changes do I need to make to my labels?
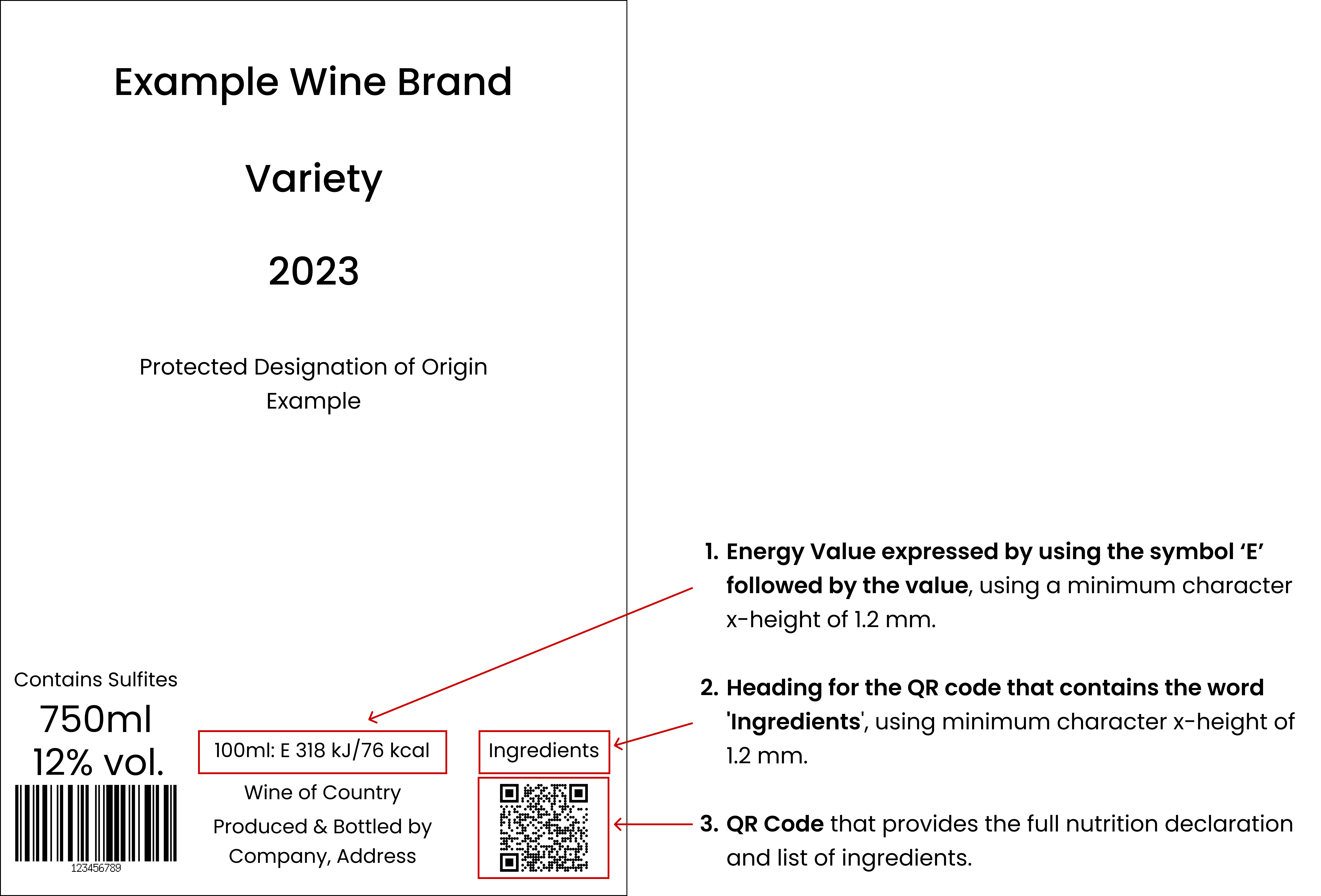
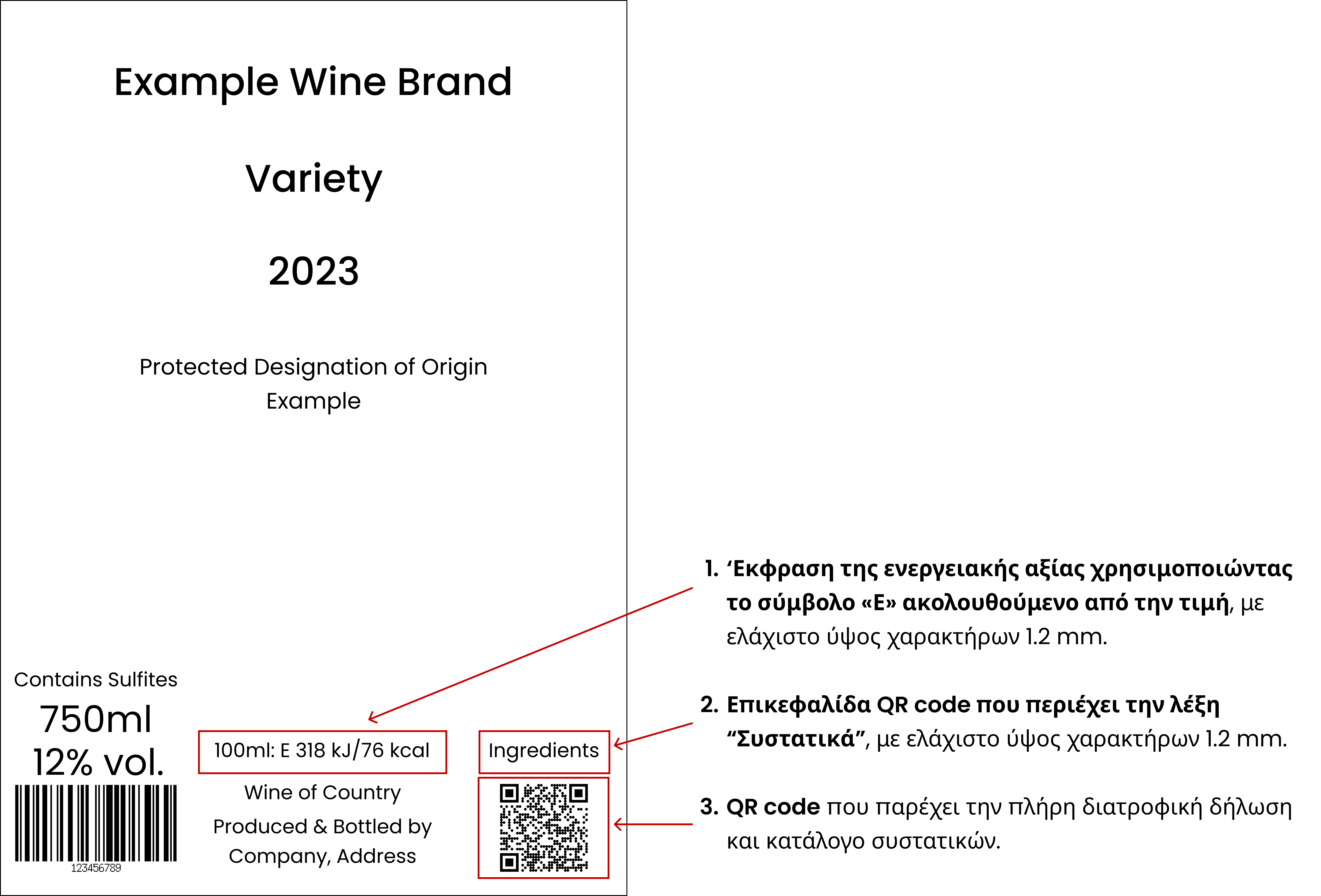
In addition to all the compulsory particulars that are required to be presented, the following have to be included on the label, in the same field of vision as the other compulsory particulars:
An indication of the Energy Value expressed by using the symbol ‘E’ followed by the value. (For example the following indication may be added: “100ml: E 318 kJ/ 76 kcal”)
A heading for the QR Code that contains the word ‘Ingredients’.
A QR Code that links to a page that presents the full Nutrition declaration and List of ingredients according to the rules set by the EU Regulations.
The presentation of the QR code should be clear for the consumers regarding its content i.e., the compulsory information that is presented by electronic means. According to EU commission notice C/2023/1190: “Where the information provided by electronic means (identified by e.g. a QR code) is the list of ingredients, a heading, as referred to in Article 18(1) of the FIC Regulation, must be used, in the same way as the current practice used for the paper labels for other food (i.e. containing the word ‘ingredients’).”
The language of the word ‘Ingredients’ may be in one (or more) of the 24 official EU languages, however it is recommended to use the a language that is easily understood by the consumers of the member state where the wine is marketed.
In any case, the height of the characters used to present the wording for the QR Code heading and the Energy Value on the label, must be equal to or greater than 1.2 mm, regardless of the character format used.
For grapevine products which have undergone a de-alcoholisation treatment and that have an actual alcoholic strength by volume of less than 10%, the date of minimum durability must also be included on the presentation of the compulsory particulars on the label.
Any ingredients or products causing allergies or intolerances must continue to be indicated on the label, even if they are already included in the list of ingredients. For example, if a wine contains sulphites, it must have a statement like “contains sulphites” on the label.
Example for the presentation of the compulsory particulars on a wine label
Tips to improve the likelihood that consumers will be able to scan your QR codes
- Test Before Final Printing: For new label designs, make sure that you test scanning the QR code on a prototype label before you commence the final printing for all the labels. Tests using different mobile phone models, light conditions and scanning distances can help capture potential issues.
- Maintain Distance Between Codes: Placing the QR code nearby other QR codes or barcodes may make it harder for the consumers to scan it. Therefore, make sure you keep a reasonable distance between them.
- Include a Quiet Zone: When downloading our QR codes, we include a margin (known as “quiet zone”) that is adequately sized to ensure the QR code’s readability across a wide range of conditions. The quiet zone is the empty space around the QR code that separates it from other visual elements and helps QR scanners distinguish the code from its surrounding environment.
- Use Required QR Code Size: Factors such as the print quality, print surface and curvature, choice of colours, mobile phone model, light conditions, scanning distance and angle can directly affect the minimum size required for a QR code to be scannable. For example, the size of the QR code directly influences the distance from which it can be scanned. As a general rule, a QR code of 1 cm x 1 cm in size corresponds to 10 cm of scanning distance but this will depend on other factors as well. Therefore, always test on a prototype label with the required conditions for your product and consumer.
- Use High Contrast: A high contrast between the QR code and its background is vital for scanability, as it helps QR scanners distinguish the code’s patterns against its surroundings. A classic example is black on white, but other combinations that provide similar visibility are also effective. For transparent labels, the colour of the wine might affect the scanability of the QR code. Therefore, always test on a prototype wine label, placed on the packaging/bottle with the contents full as well as empty.
- Use High Quality Printing: We recommend printing at a resolution of at least 300 DPI to help maintain the integrity of the QR code’s design, enabling scanners to accurately read the code even at smaller sizes.
DISCLAIMER: The information contained in this document represents our interpretation of the regulatory requirements. Whilst due care and diligence has been exercised in preparing this guide, it is not intended to be a substitute for legal advice and should not be relied upon as such. Information provided may not be completely accurate as regulations may have changed since its publication or because clear information is not available. We strongly recommend consulting official sources and documents for further guidance. We disclaim all and any liability and responsibility to any person in respect of the consequences of anything done in respect of reliance, whether wholly or in part, upon this document.